Gender-specific personalised medicine: When viruses awaken the hormones

Gisela Köhler/LIV
Professor Gülşah Gabriel heads the Department of Viral Zoonoses at the Leibniz Institute of Virology in Hamburg.
What lessons can be learned from COVID-19 for future pandemics? Researcher Gülşah Gabriel and her team are opening the door to personalised medicine – and are investigating for the first time how drugs that intervene in hormone metabolism could in future be used to prevent severe viral progression.
It all began in 2013, long before the global coronavirus pandemic, with a mysterious phenomenon in China. Back then, significantly more men than women fell seriously ill during the spread of the H7N9 avian influenza virus, better known as bird flu. A curious finding, because until then, hardly anyone had investigated whether and, if so, how, viral infections differ between men and women.
What we discovered in the human lung was mind blowing.
'That was our first lead at the time,' says Prof Gülşah Gabriel, who heads the Department of Viral Zoonoses at the Leibniz Institute of Virology in Hamburg. Gabriel recalls: 'At the time, we explained the findings quite simply by saying that men come into contact with live poultry more frequently in the chain of production.' But this explanation alone proved to be insufficient: 'In the years that followed, it also emerged that around 70 per cent of those infected who subsequently became seriously ill were men. This could no longer be explained away by the greater frequency of contacts.' What was the real reason?
The virologist's team found the answer years later, when men again fell ill more frequently and more severely during the coronavirus pandemic. 'We realised that a completely different mechanism must be playing a key role here.' But what was it?
The decisive breakthrough came with the discovery of an enzyme known as aromatase. After infection with SARS-CoV-2, it is suddenly produced in greater quantities in the lungs. 'The lungs become an endocrine organ that converts hormones – more precisely, testosterone into oestradiol,' explains Gabriel. This was a ground-breaking discovery, as the lungs normally have hardly any hormonal functions. A whole new branch of research was born: infection endocrinology. In this field, infection researchers are working together with endocrinologists to investigate how infections affect hormone systems and what consequences this has for the course of the disease.
The lungs as a hormone factory
'What we discovered in the human lung was mind blowing,' says Gabriel. Autopsy studies on COVID-19 deaths in Hamburg, Tübingen and Rotterdam showed that aromatase was induced ten to fifty times more strongly than usual in men – a finding that correlated with low testosterone and elevated oestradiol levels in the blood. This effect did not occur in women. They simply lacked the substrate, as they have hardly any testosterone anyway.
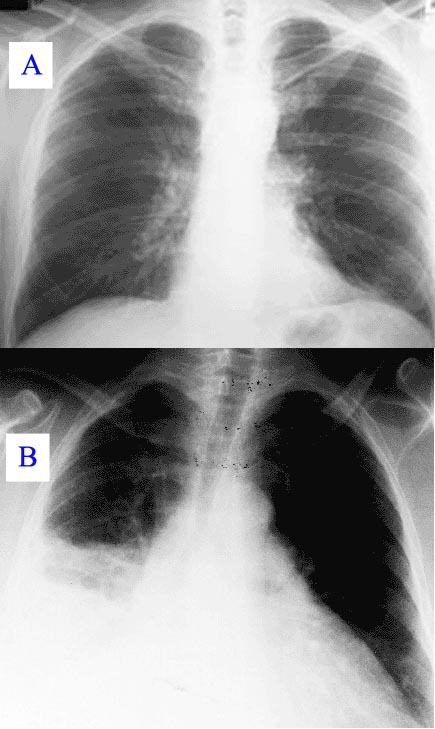
X-ray image: healthy lung (top) and a lung with pneumonia (bottom) in comparison.
The newly created hormone factory had dramatic consequences: 'It led to more severe disease progression in men,' explains Gabriel. The more oestradiol, the worse. But were the two findings actually causally related? Did one cause the other? To test this, Gabriel and her team turned to a hamster model. 'We then saw that male hamsters become more severely ill after aromatase induction and exhibit poorer lung function in the long term due to scarring of the lungs,' explains Gabriel. Oestradiol apparently activates inflammatory signals and thus leads to long-term damage to the lung tissue. In contrast, this effect was not observed in the female animals. 'This made aromatase the first gene ever to be associated with severe lung disease in men. A real novum.'
Instead of attacking the virus directly [...], we focus on what it triggers in the body and try to mitigate the consequences of the infection.
But how can this fatal effect be prevented? This is precisely where Professor Gülşah Gabriel's 'FLU-FLAME' research project comes in. The aim is to identify drugs that do not combat the virus itself, but rather the harmful consequences of the infection – by inhibiting aromatase, for example. 'We were able to show that the aromatase inhibitor letrozole, for example, which is already used in cancer therapy, can actually improve lung function in male animals over the long term,' says Gabriel.
A completely new approach: 'Instead of attacking the virus directly – which is difficult because viruses mutate so quickly – we focus on what it triggers in the body and try to mitigate the consequences of the infection.' The coronavirus pandemic has shown that it is not enough to simply develop antiviral drugs or vaccines – both need time before they are available on a large scale.
Therapeutic approaches for the future
On the other hand, aromatase, but also oestrogen receptors offer stable targets. Drugs with these targets have already been clinically tested and could play a key role as preventive, rapidly deployable therapies. 'This could potentially reduce severe and long-lasting lung damage in future respiratory pandemics, such as those caused by avian influenza viruses, which are currently considered the greatest zoonotic threat,' emphasises the researcher.
This type of medicine is long overdue
Bird flu viruses in particular appear to have the dangerous ability to strongly induce aromatase in the lungs, which suggests a significantly increased risk of long-term lung damage – a consequence that has hardly been researched to date. 'Today's acute viral infections could lead to tomorrow's chronic diseases,' says Gabriel. 'We can see that the inflammatory processes triggered by the viral infection are sometimes still active several months after the infection.' Accordingly, surviving a bird flu infection can have just as long-lasting consequences as a coronavirus infection. 'We are already talking about "long influenza" in scientific circles. This could become a major health problem in the future.'
The 'FLU-FLAME' research project thus represents the first major step in the innovative field of "pandemic preparedness". 'Interdisciplinary collaboration is essential in order to meet the challenges of future pandemics at an early stage,' says Gabriel. The project is currently in the decisive preclinical phase. Initial results are promising and clinical trials are to follow soon – another milestone in the preparation for future influenza pandemics.
In the best-case scenario, Gabriel's research results will lead to gender-specific personalised medicine. 'This type of medicine is long overdue', says Gabriel. She emphasises how important it is to take the different hormonal and biological conditions of women and men seriously – a perspective that could be crucial in any future pandemic.
Gabriel's team has opened the door to a new era in pandemic research, with the vision of better treating or even preventing acute and chronic consequences of viral respiratory diseases in the future. It remains exciting to see what new findings will emerge from this promising field of research – not least in order to be prepared for the next pandemic. Because one thing seems certain: the question is not if it will come, but when.

